This table contains information on growth, natural mortality and length at first maturity, which serve as inputs to many fish stock assessment models. The data can also be used to generate empirical relationships between growth parameters or natural mortality estimates, and their correlates (e.g., body shape, temperature, etc.), a line of research that is useful both for stock assessment and for increasing understanding of the evolution of life-history strategies (see Fig. 19).

Fig. 19. Auximetric plot for Sardinella longiceps and of 20% of the data points for other species.
The growth parameters included in this table are those of the von Bertalanffy Growth Function (VBGF; von Bertalanffy 1938), which takes for growth in length the form
![]() ...1)
...1)
where L
Similarly, the VBGF for growth in weight takes the form
where Wt and W¥
are the weights corresponding to L
W = a
POPGROWTH includes records for which at least L¥
and K are available, i.e., t0 may be absent (this non-biological parameter is not required for most stock assessment models).
![]() ...2)
...2)
![]() ...3)
...3)
The table presently contains over 5,000 sets of growth parameter estimates for over 1,300 species, extracted from about 2,000 primary and secondary sources. The compilations of Pauly (1978, 1980) contributed about 1/4 of the entries.
In addition to the MainRef., a data Ref. is given for each set of growth parameters, as these are often presented in papers that do not include the data from which the estimates were derived. The ‘source data’, as indicated by a choice field, may consist of: otolith annuli; scale annuli; other annual rings; daily otolith rings; tagging/recaptures; length-frequencies; direct observations; several data types; others.
Also, the method used to estimate a given set of growth parameters is recorded, through selection from a choice list consisting of the following items: Ford-Walford plot; von Bertalanffy/Beverton plot; Gulland and Holt plot; Nonlinear regression; ELEFAN I; other method(s).
Accounts of these methods and their assumptions and biases, and of their data requirements may be found in Ricker (1975), Gulland (1983), Pauly (1984), Gayanilo and Pauly (1997), and other fisheries science texts.
To verify the gross accuracy of growth parameters we included the following:
a calculated field with the growth performance index ø' = log10K + 2log10L¥ (Pauly 1979; Pauly and Munro 1984 and see ‘Auximetric Analyses’, this vol.), which can be compared with ø' values for other stocks of the same, or closely allied species;
a multiple choice field describing how L¥ was converted into W¥ , with choices as follows:
As given in MainRef. or Ref. for growth;
Computed using L/W rel. of same stock;
Using L/W rel. of other stock of same species;
Computed using L/W rel. of similar species;
Other (see Comments).
a yes/no field is used to identify cases in which L¥
differs from Lmax (in the SPECIES table) by more than 30% of Lmax;a yes/no field indicating, when n > 4 records are available, whether a given pair of W¥ , K values fall outside of the auximetric ellipse (see ‘Auximetric Analyses’, this vol.) defined by the other W¥ , K records for the species in question;
a graph button in the summary table which, upon clicking, displays plots of body length on relative age (Fig. 20), and which can be used to identify growth curves that deviate from the general trend;

Fig. 20. Body length vs. relative age (t-t0) in Oreochromis niloticus niloticus. These curves are
based on the parameters L¥
and K in the POPGROWTH table, and the VBGF (Equation 1). The growth curves with low asymptotes tend to reflect growth in captivity (see Box 16 and
Fig. 21).
graph buttons to display auximetric plots, i.e., plots of logK vs. logL¥ (see Fig. 19) or W¥ (see ‘Auximetric Analyses’, this vol.);
a field to indicate whether a set of growth parameters originate from fish in ‘open waters’ or in ‘captivity’ (see Box 16).
Information on length at first maturity, which also appears in a separate table (MATURITY), is used here in conjunction with L¥ to compute the ‘reproductive load’ (Cushing 1981) of the population, i.e., the ratio Lm/L¥ . Most of the Lm values refer to mean length or the length at which 50% of the population become mature, but when such estimates were not given, or could not be derived from the data, Lm was taken as the midrange of published values.
In open waters, environmental conditions (e.g., temperature, but also the presence of predators), cause fish to either grow rapidly toward a small size (high K, low L¥ ), or leisurely toward a large size (low K, high L¥ ). This leads to their growth performance index (ø
’ = logK + 2 log L¥ ) remaining nearly constant among different populations of the same species (Pauly 1994). The reasons for this near constancy of ø’, which is ultimately due to the way fish allocate the scarce oxygen diffusing through their gills, are discussed in Pauly (1981, 1994).
For most captive fish, the absence of predators and sexual competitors allows the allocation of more oxygen to feeding and growth, and away from behaviors that are costly in terms of oxygen demand, such as darting about to evade predators, or fighting against sexual competitors.
This results in captive fish usually having ø
Combined, these effects cause the ø
As might be seen on the auximetric plot in Fig 21, the dots pertaining to captive fish form a cluster that deviates strongly from a cluster representing their wild conspecifics, especially for L¥
values between 10 and 30 cm, mostly representing Nile tilapia in intensive systems.
References
Bozynski, C.C. 1998. Interactions between growth, sex, reproduction, and activity levels in control and fast-growing strains of Nile tilapia (Oreochromis niloticus). Department of Zoology, University of British Columbia. Master thesis. 109 p.
Gjedrem, T. 1985. Improvement of productivity through breeding schemes. Geo J. 10(3):233-241.
Jones, R.E. 1996. Comparison of some physical characteristics of salmonids under culture conditions using underwater video imaging techniques. University of British Columbia. Master thesis. 104 p.
Pauly, D. 1981. The relationships between gill surface area and growth performance in fish: a generalization of von Bertalanffy's theory of growth. Meeresforschung 28(4):251-282.
Pauly, D. 1994. On the sex of fish and the gender of scientists: essays in fisheries science. Chapman and Hall, London. 250 p.
Pauly, D., J. Moreau and M. Prein. 1988. Comparison of growth performance of tilapia in open water and aquaculture, p. 469-479. In R.S.V. Pullin, T. Bhukaswan, K. Tonguthai and J.L. Maclean (eds.) Proceedings of the Second International Symposium on Tilapia in Aquaculture, 16-20 March 1987, Bangkok, Thailand. ICLARM Conf. Proc. 15.
Pullin, R.S.V., Editor. 1988. Tilapia genetics for aquaculture. ICLARM Conf. Proc. 16, 108 p.
Daniel Pauly
For some records, the estimates of L¥ have yet to be checked against the recorded maximum length (Lmax) to which, we believe, L¥ should be reasonably close (see above).
Data in this table have contributed to a recent study on empirical equations for important parameters such as L¥
, length at first maturity, and length at optimum yield (Froese and Binohlan 2000).
We look forward to users’ comments on the contents and/or utility of the POPGROWTH table.
Life-history theory, which is of high importance to both theoretical ecology and resource management, is based on the concept of trade-offs between different energy-consuming functions, and the resulting balance tends to maximize fitness (i.e., total reproductive output; e.g., Beverton 1963, Roff 1992, Stearns 1992, Charnov 1993). FishBase can be very useful for testing life-history hypotheses and identifying patterns at a large geographical scale (e.g., Natural Mortality, this vol.). An example of such a use at a small geographical scale is presented here. Stergiou et al. (1997) reviewed the available quantitative information on the physics, chemistry, biology and fisheries of the Greek Seas. The available data clearly indicate the highly oligotrophic nature of the subtropical Greek waters, with large areas being directly comparable, in terms of trophic potential, to open oceans. Since temperature and the quality and quantity of food are among the most important factors affecting phenotypic responses in fishes (e.g., Wootton 1990, Roff 1992), one may predict, that the fish stocks and/or species inhabiting Greek waters will be generally smaller in size, have lower longevity, mature at an earlier age and size, and probably suffer higher adult mortality rate than their counterparts in other areas of the world (for a discussion on the relationship between trophic potential, temperature, growth rates, body sizes, predatory fields and adult natural mortality rates, and length at maturity see, Pauly 1980, and Natural Mortality, this vol.).
To test the prediction of smaller sizes, the relationship between the VBGF parameters K and L¥
of the various fish stocks reviewed by Stergiou et al. (1997) was estimated, and L¥
-K pairs were plotted against those of all stocks included in FishBase 98 (Fig. 22). The following relationship was established: LogL¥
=1.34-0.32LogK (SE-slope = 0.12, r = - 0.25, n = 99, P<0.05). The slope of the LogL¥
-LogK relationship in Greek waters was significantly (ANCOVA, P<0.05) smaller than that for all records included in FishBase 98, excluding those which refer to fish in captivity, and for which von Bertalanffy estimates are available: LogL¥
=1.33-0.61LogK (SE-slope = 0.009, r = -0.70, n = 4,618, P<0.001; Fig. 22). From Fig. 22, it is evident that the Greek stocks are characterized, for the same K values, by smaller L¥
values (i.e., the vast majority of the points are positioned below the ‘global’ FishBase regression line), for lengths up to 100 cm (i.e., LogL¥
about 2). The only notable exceptions were the seven Xiphias gladius stocks to the right (Fig. 22), the removal of which did not affect the slope of the ‘Greek’ regression line (i.e., LogL¥
=1.29-0.30LogK, SE-slope=0.09, r=-0.32, n=92, P<0.05). X. gladius does not follow the general trend mentioned above because it is a highly migratory species and thus its growth is most probably less affected by local environmental (i.e., food, temperature) conditions.
References
Beverton, R.J.H. 1963. Maturation, growth and mortality of clupeid and engraulid stocks in relation to fishing. Rap. P. V. Réun. Cons. int. Expl. Mer 154:44-67.
Charnov, E. L. 1993. Life history invariants: some explorations on symmetry in evolutionary ecology. Oxford Series in Ecology and Evolution. Oxford University Press, Oxford. 182 p.
Pauly, D. 1980. On the interrelationships between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks. J. Cons. int. Explor. Mer 39:175-192.
Roff, D.A. 1992. The evolution of life histories: theory and analysis. Chapman and Hall, London.
Stearns, S.C. 1992. The evolution of life histories. Oxford University Press, Oxford. 262 p.
Stergiou, K.I., E.D. Christou, D. Georgopoulos, A. Zenetos and C. Souvermezoglou. 1997. The Hellenic seas: physics, chemistry, biology and fisheries. Oceanogr. Mar. Biol. Ann. Rev. 35:415-538.
Wootton, R.J. 1990. Ecology of teleost fishes. Fish and Fisheries Series 1. Chapman and Hall, London. 404 p.
Konstantinos I. Stergiou
You get to this table by clicking on the Biology button in the SPECIES window, Population dynamics button in the BIOLOGY window, and Growth button in the POPULATION DYNAMICS window. Fig. 21, emphasizing the growth of captive fishes (e.g., in aquaculture experiments) may also be accessed through the Population dynamics button of the Graphs Menu accessed via the Reports button of the FishBase Main Menu.
On the Internet, you get to the POPGROWTH table by clicking on the Growth link in the ‘More information’ section of the ‘Species Summary’ page. Alternatively, you can create a list of all species for which growth information is available by selecting the Growth radio button in the ‘Information by Topic’ section of the ‘Search FishBase’ page.
Cushing, D.H. 1981. Fisheries biology: a study in population dynamics. 2nd ed. University of Wisconsin Press, Madison. 295 p.
Froese, R. and C. Binohlan. 2000. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 56:758-773.
Gayanilo, F.C., Jr and D. Pauly. 1997. The FAO-ICLARM Stock Assessment Tool (FiSAT). Reference Manual. FAO Comp. Inf. Ser./Fish. 8, 262 p.
Gulland, J.A. 1983. Fish stock assessment: a manual of basic methods. FAO/Wiley, New York.
Pauly, D. 1978. A preliminary compilation of fish length growth parameters. Ber. Inst. Meereskd. Christian-Albrechts Univ. Kiel 55, 200 p.
Pauly, D. 1979. Gill size and temperature as governing factors in fish growth: a generalization of von Bertalanffy’s growth formula. Ber. Inst. Meereskd. Christian-Albrechts Univ. Kiel 63, 156 p.
Pauly, D. 1980. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J. Cons. CIEM 39(2):175-192.
Pauly, D. 1984. Fish population dynamics in tropical waters: a manual for use with programmable calculators. ICLARM Stud. Rev. 8, 325 p.
Pauly, D. and J.L. Munro. 1984. Once more on growth comparison in fish and invertebrates. Fishbyte 2(1):21.
Ricker, W.E. 1975. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 191, 382 p.
von Bertalanffy, L. 1938. A quantitative theory of organic growth. (Inquiries on growth laws II). Hum. Biol. 10:181-213.
Box 17. Using FishBase to test life-history hypotheses.
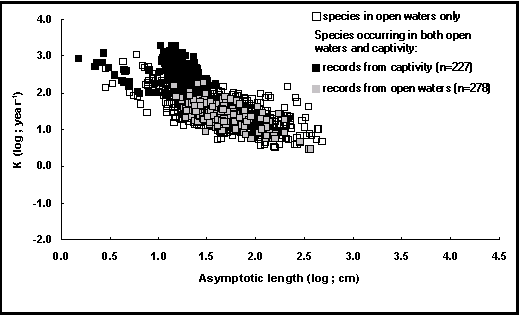
Fig. 21. Auximetric grid, emphasizing the growth of captive fishes. The cluster of black squares between log(L¥
) =
1.0-1.5 refers mainly to Nile tilapia in semi-intensive and intensive systems (see Box 16).
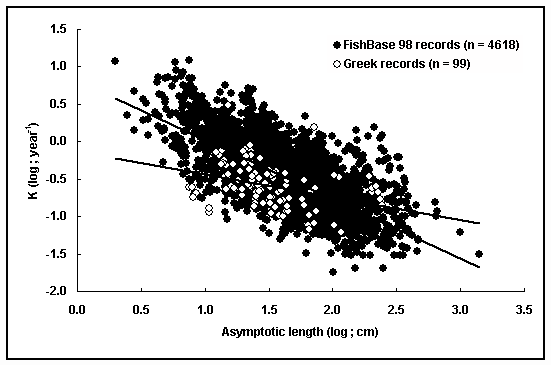
Fig. 22. Relationship between the von Bertalanffy parameters K and L¥
for a variety of fish stocks in Greek waters and for
records available in FishBase 98 (excluding those which refer to fish in captivity). The two slopes differ significantly
(ANCOVA, P< 0.05).
How to get there
Internet
References