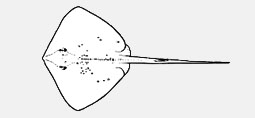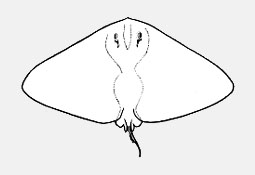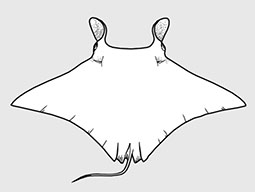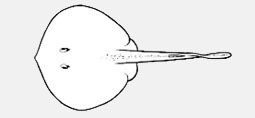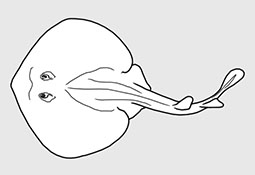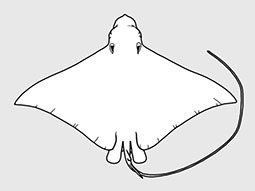
Aetobatidae - (Pacific eagle rays)
Distribution: Atlantic, Indian and Pacific Oceans. Chiefly marine; also in brackish and freshwater. Diagnosis: small to very large; body variably depressed, a well-formed oval, circular or rhombic disc, fully incorporates head; snout angular to obtuse, sometimes very elongate; nasal curtain well developed, skirt-shaped, rectangular or bilobed; gill slits 5; oral papillae usually present on floor of mouth; tail moderately stout to slender-based, more or less elongated (sometimes very elongate and whip-like); dorsal surface variably covered with dermal denticles, thorns and/or tubercles, smooth to very spiny, often with a median thorn row and/or a median denticle band; absence of dorsal or caudal fins; prominent caudal stings 1-4, on tail well posterior to pelvic fins; skin folds variably developed, on ventral and sometimes dorsal midline of tail; dorsal surface plain to strongly patterned, often darker than ventral surface. Max size: 22-260 cm DW (adults) Subfamilies: Dasyatinae Jordan and Gilbert, 1879 - no unique character states; disc circular, flattened cone-shaped but mostly rhombic; snout short to long; tail variably depressed, short and firm to long and whip-like; oral papillae 1-7 or none; dorsal tail fold developed or reduced to a fleshy ridge or none; no denticle band (typically weak if present), no tail thorns in most species, usually plain dorsal surface coloration with dark posterior tail; all members with a short to long-based ventral tail fold that can be poorly developed to deep; caudal sting relatively well forward on tail with distance from pectoral-fin insertion to caudal-sting base 2-3 times interspiracular width. - Genera: Bathytoshia, Dasyatis, Hemitrygon, Hypanus, Megatrygon, Pteroplatytrygon, Taeniurops, Telatrygon. Hypolophinae Stromer, 1910; Neotrygoninae Last, Naylor & Manjaji-Matsumoto, 2016; Urogymninae Gray, 1851.
Distribution: Atlantic, Indic and Pacific Oceans. Marine, rarely in estuaries. Outer anterior margin of pectorals continuous along side of head. Disc extremely broad. Dorsal fin and tail spines present or absent. Tail short.
Hexatrygonidae - (Sixgill stingrays)
Distribution: off South Africa. Snout translucent, depressed, produced and may act as an electroreceptive organ. Gill openings 6; gill arches 6. Cranium without crests above the eyes. Very small brain in posterior of large cranium. Large spiracles with external flaplike valve and well behind eyes. Two serrate spines in tail. Length of disc greater than width. Nostrils widely set, with short anterior nasal flaps.
Distribution: circumglobal in tropical and warm temperate seas. Habitat: mostly pelagic over continental shelves and near offshore islands; often in small to very large groups and mostly observed swiiming neat the water surface. Traits and feeding: vivparous (aplacental), one pup per litter; feed mainly on planktonic organisms sieved from the water using the highly modified branchial filter plates.. Morphology: Medium to very large rays (adults attain 1.1 m to at leaset 7 m in disc width), with a rhomboidal and 'wing-like' disc that is much broader than long; head broad protruding forward anteriorly beyond eye level and with prominent cephalic lobes extending forward on each side; broad trunk depressed and thick; location of eyes and spiracles lateral; mouth very broad, either terminal or ventral (subterminal), jaws with minute tooth bands or only in the lower jaw; tail long to relatively short, usually much less than dsic width; small dorsal fin near its base; sometines with a small serrated caudal sting; skin sometimes rough but without thorns or enlarged denticles (Ref. 114953). Uses: caught mainly as bycatch in several tropical net fisheries; when targeted in some areas, for the filter plates or human consumption; mostly discarded at sea or made into fishmeal. Important to diving ecotourism.
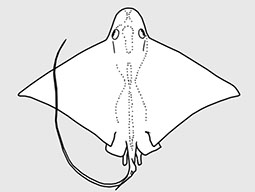
Myliobatidae - (Eagle and manta rays)
Atlantic, Indian, and Pacific Oceans. Head elevated above disc; jaws powerful with large platelike crushing teeth in several rows in eagle rays; eye and spiracles lateral on head; gill openings about length of eye to much longer; tail much longer than disc; venemous spine(s) present in some; small dorsal fin; pectoral fins reduced or absent opposite the eyes, but with an anterior subdivision that unites below the tip of the snout forming a subrostral lobe in manta rays. Some known for their leaping ability high into the air. Viviparous with 2-6 fully developed young. Plankton-filtering manta rays are among the largest fishes, but harmless.
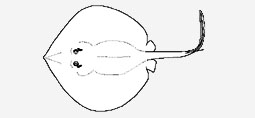
Plesiobatidae - (Deepwater stingrays)
Indo-Pacific: off Mozambique, Hawaiian Islands, and possibly southern China. Nasal curtain incompletely united, not reaching the mouth. Maximum length 2.7 m. Family established by Nishida (1990) with the single species Plesiobatis daviesi, formerly assigned to the genus Urotrygon.
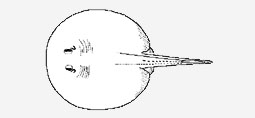
Potamotrygonidae - (River stingrays)
Neotropical freshwater stingrays of the family Potamotrygonidae presently comprise three genera (Potamotrygon, Paratrygon and Plesiotrygon) and at least 18 species. The family has an extensive taxonomic history dating back to 1834, but was known long before formal taxonomic recognition (Castex, 1963a). Potamotrygonids are much maligned and feared because of their venomous caudal stings, but pose little or no threat if not stepped on or directly interfered with. The Potamotrygonidae is the only living chondrichthyan family restricted to freshwater habitats. Potamotrygonid stingrays are clearly monophyletic, sharing unique morphological and physiological specializations, including a pelvis with a greatly expanded anterior median process (pre-pelvic process), blood with low concentrations of urea, and reduction of the rectal gland (Garman, 1877; Thorson et al., 1983a). They are generally medium to large sized batoids, ranging from about 25 cm in disc width or length to more than 100 cm in adults of some species. Species of Potamotrygon have moderately stout and short tails, usually shorter than disc length, whereas Paratrygon and Plesiotrygon have slender, filiform and whiplike tails (in Paratrygon the tail is very long in juveniles but absolutely reduced in adults, and in Plesiotrygon it is much longer than disc length but also stout at base). The dorsal surface of the disc and tail are usually covered with many denticles, thorns and tubercles. The caudal sting (or serrated spine) is a rigid dermal derivative, located on the dorsal surface of the tail, containing small lateral serrations directed toward its base and an acute distal tip. The sting is reduced and situated closer to the tail base in Paratrygon, but well developed and located farther posteriorly in both Plesiotrygon and Potamotrygon. The stings contain longitudinal grooves to conduct venom produced in special glands at their bases, and are continuously worn, shed and replaced; up to four stings may be present in one individual. Additionally, many species have enlarged, non-venomous spines on disc margins or over tail, sometimes in numerous rows. The disc is usually slightly longer than wide (especially in Paratrygon), and covers most of the pelvic fins posteriorly (but less in Plesiotrygon). Dorsal and caudal fins are absent, but membranous skin folds (finfolds), with rudimentary internal radial elements, occur in Potamotrygon on both upper and lower tail midlines posterior to caudal stings (only ventral finfold is present in Plesiotrygon, and finfolds are absent in Paratrygon). The eyes are moderately large in Potamotrygon, but smaller and less protruded in Paratrygon and Plesiotrygon. Oral teeth are small with short cusps (more prominent in adult males), in usually less than 50 rows in either jaw (large specimens of Plesiotrygon may have more than 60 rows), and set in quincunx. Most potamotrygonid species have colorful dorsal arrangements, including spots of various dimensions, ocelli, reticulate patterns, and vermiform markings, which are generally species-specific, and grey, brown or black background coloration. Potamotrygonids are ovoviviparous (aplacentally viviparous), and the developing embryos are nourished by uterine milk secreted by trophonemata (Thorson et al., 1983b). Both uteri are functional and appear to be synchronous. Gestation may be restricted to certain stations or occur throughout the year, and the number of young produced in each gestation varies among species, but is usually from two to seven. Potamotrygonids only occur in South American rivers that drain into the Atlantic Ocean or Caribbean Sea. They are are conspicuously absent, however, from the São Francisco basin in northeastern Brazil, rivers that drain into the Atlantic from the Atlantic rainforest of northeastern and southeastern Brazil, the upper Paraná basin, and rivers south of the La Plata river in Argentina. Generally, most potamotrygonid species have distributions restricted to a single basin or river system, with only a few species present in more than one basin (e.g. Potamotrygon motoro, Potamotrygon orbignyi). Some species are even restricted to a single river (e.g. Potamotrygon leopoldi). This high endemism has led recent workers to express concern that some species may be endangered (Compagno & Cook, 1995; Rosa & Menezes, 1996), or are at least clearly vulnerable at present (two species are cited on the IUCN Red List as "data deficient"). Potamotrygonids are generally not consumed as food, but are commercialized in increasing quantity by the aquarium trade, and are only seldomly bred in captivity for commercial purposes. Further studies on their population structure and dynamics are therefore very much needed. The literature concerning potamotrygonids is vast. After the revision of Garman (1913), the next taxonomic study to treat the entire family is that of Rosa (1985a). Anatomical descriptions can be found in Garman (1877, 1913), Thorson & Watson (1975), Rosa (1985a), Nishida (1990), Taniuchi & Ishihara (1990), and Lovejoy (1996). Their phylogenetic relationships have been treated by Rosa (1985a), Dingerkus (1995), Lovejoy (1996) and McEachran et al. (1996). Accounts of their natural history are presented by Castex (1964a) and Castello (1975). Physiological aspects of potamotrygonids are summarized in Thorson et al. (1983a), and the reproductive biology of certain species by Thorson et al. (1983b), Teshima & Takeshita (1992), and Lasso et al. (1997a). Their historical biogeography has been studied by Brooks et al. (1981), Lovejoy (1996, 1997), Lovejoy et al. (1998), and Lundberg (1998). Recent descriptions of fossil remains (mostly isolated denticles) can be found in Deynat & Brito (1996) and Lundberg (1997). Recent taxonomic and historic compilations are those by Compagno & Cook (1995) and Zorzi (1995), and the extensive literature on potamotrygonid parasites is summarized in Brooks & Amato (1992). The following account is mostly based on original work by the first author, supplemented by information from Rosa (1985a) concerning type specimens of Potamotrygon deposited in museums in Argentina. This account differs from the revision of Rosa (1985a) in relation to the fate of various nominal species, and is also more conservative, recognizing fewer species. At least five new species have been found and are not included here (these are presently being described elsewhere). Taxonomic work on the family is constrained by the fact that many species of Potamotrygon are poorly described, lack adequate material, or present much intraspecific variation in coloration. This is compounded by generally overlapping meristic and morphometric features among species of Potamotrygon (both other genera are monotypic). Therefore, the approach adopted here has been to not recognize certain species that are inadequately defined at present, or that lack adequate material for proper characterization (e.g. Potamotrygon dumerilii,Potamotrygon humerosa). The current account is a working summary of the family, one that is subject to modifications as more collecting and research is conducted. This family is treated as it has traditionally been used in the literature, i.e. restricting it to Potamotrygon, Paratrygon and Plesiotrygon only, and refrain from including other genera that have recently been hypothesized as being their sister-taxa (amphi-American Himantura and Taeniura; Lovejoy, 1996; McEachran et al. 1996). Note that DW is disc width, and DL is disc length, which are standard measurements in stingrays (total length is usually not used as the distal tip of the tail is frequently missing in specimens).
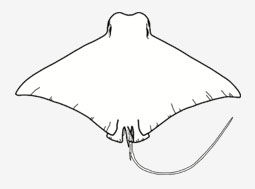
Rhinopteridae - (Cownose rays)
Distribution: circumglobal in tropical and warm temperate seas. Habitat: benthopelagic on continental shelves and near offshore islands, as well as in bays and estuaries. Morphology: Medium to large-sized rays (adult 90 cm to 1.7 m DW); disc broad and rhomboidal, ‘wing’like’, much wider than long; head distinct and narrow, protruding forward anteriorly well beyond eye level; snout strongly indented anterioryly with 2 large, fleshy cephalic-fin lobes in front of a fringed nasal curtain; trunk broad, depressed and thick; eyes and spiracles positioned laterally on head; mouth located ventrally and almost as broad as head, with plate-like teeth usually in 7 (rarely 5 ot 6) or more rows in each jaw; pectoral fins originate below spiracles; tail filamentous distally and without a caudal fin, normally longer than the disc (although undamaged tail length varies among species); small dorsal fin followed by one or more short serrated spines located near tail base; skin smooth or finely granular, no thorns nor enlarged denticles; the species under this family are so morphologically similar that it is difficult to distinguish one from another (Ref. 114953). Diet: opportunistic generalist feeders able to use suction to ingest small prey or crush hard-bodied shellfishes using their strong tooth plates. Reproduction: Viviparous but no placental connection between mother and young; most species produce a single young per pregnancy. Uses: Often occur in large aggregations that they tend to be caught in batches and appear irregularly in fish market. Caught as bycatch in tropical net fisheries and in some areas are used for human consumption. Survive well in captivity and considered as prized exhibiles in many large aquaria and oceanaria.
Atlantic, Indian and Pacific Oceans. Well-developed caudal fin; tail moderately long; outer anterior margin of pectorals continuous along side of head. Most species with one or more long poisonous spines on tail.
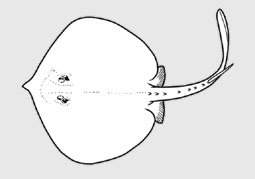
Urotrygonidae - (American round stingrays)
Distribution: Tropical to warm temperate in continanental shelves of Western Atlantic and Eastern Pacific; demersal inshore, rarely deeper than 90.. Habitat: some venture into estuaries, none considered to live entirely in freshwater. Short description: small rays (17-90 cm TL as adults, weighing at least 2.2 kg); oval to almost circular disc, variably depressed; head barely distinguishable from the part of the disc formed by the pectoral fins; snout varies from very short and obtuse to long and pointed, mouth positioned forward on the ventral disc, jaws have rows of small flattened to pointed teetharranged in quincunx; small fleshy papillae usually on the mouth floor; tail relatively broad based, usually shorter than the disc, and no dorsal and anal fins; well-developed caudal fin with short dorsal and longer ventral lobes, varies from short to very elongate; often have serrated stinging spine preceding caudal fin, sometimes with a narrow skin fold on each side of tail; pelvic fins at the base of the tail; skin may be entirely smooth to covered with small denticles and/or thorns dorsally. Previously in Urolophidae (stingarees), but now known to be closely related to Potamotrygonidae (Neotropical stimgrays). Life history: aplacental viviparous, 1-6 pups that usually gestating over ca. 6 months. Caught as bycatch by inshore trawlers, beach seines and set nets
Distribution: Eastern Atlantic, off Western Africa. Habitat: coastal on continental shelf; from shallow waters inshore to ca. 100 m depth. Morphology: size small to moderate (to ca. 60 cm TL); large pectoral fins forming a heart-shaped or subcircular, flattened disc; short and bluntly angled snout; tail slender, depressed and demarcated from disc; eyes on top to oval,no cusps on their crowns; nostril well-developed, just forward of mouth, connected to it by groove; nasal flaps (anterior nasal valves) are enlarged and cornet-shaped; body covered densely with tiny dermal denticles giving the skin a silky feel; clusters of thorns and thornlets on snout, around orbits, nape and shoulder area, in concentric rows on pectoral fins and in mid-dorsal row on trunk and tail; pectoral fins are developed and exposed in dorsal view (pectoral postrior margin covering pelvic-fin origins); posteior tail with 2 small and equal-sized dorsal fins, each with rounded apex and convex posterior amrgin; origin of first dorsal-finwell behind free rear tip of pelvic fins; elongated and rounded caudal fin without distinct lower lobe. Colour of dorsal surface brownish or greenish with variegated pattern of dark brown transverse bars and/or blotches, sometines with some white or black spots; ventral surface pale, with ot without dark posterior and pelvic-fin margins, sometimes with a few dark blotches scattered on the belly, sometimes darker oronasal an gill areas. Trophic ecology: bottom-dwellers, feeding on small invertebrates including crustaceans, worms and molluscs. Reproduction: ovaviviparous with only the right oviduct functional producing 1-4 pups after gestation period or about 5 months. Uses: caught as bycatch of bottom trawls and gillnets; meat not utilized because they are extremely difficult to skin and discarded by fishermen.
Note: Families with unknown counts of dorsal or anal spines are also included